American company Merck and Ridgeback Biotechnics have made the first tablet available for the treatment of coronavirus.
The covid-19 pill manufactured by Merck has been approved by the UK Medicines and Healthcare Products Regulatory Agency. The Merck company claims that the tablet showed better results in clinical trials last month.
The pill reduces the risk of death or hospitalization with covid-19 by 50 percent.
Sajid Javid, Britain’s health secretary, said the government was working with the Department of Health Services (NHS) to study the country as soon as possible and formulate plans to provide the covid-19 pill to patients.
Britain became the first country in the world to approve a tablet for coronavirus treatment.
Read More: Ignoring Oral Healthcare Can Lead To These Serious Health Issues
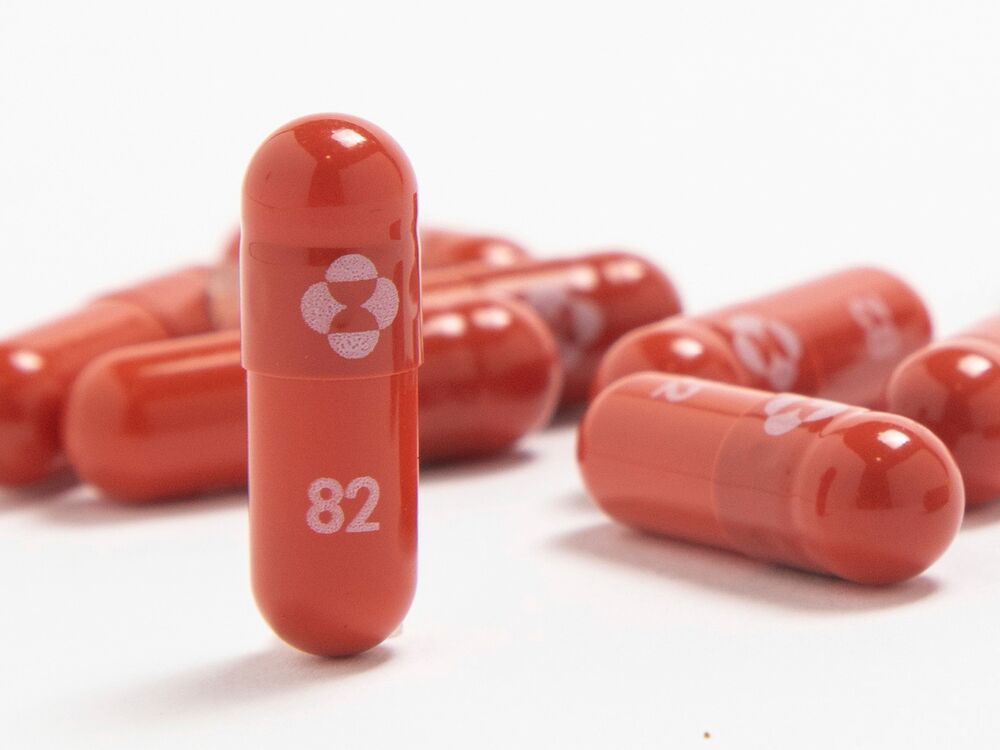
This is the first oral antiviral treatment for COVID-19 to be approved, with the approval coming ahead of potential regulatory approval in the United States. This month, US advisers will meet to vote on whether molnupiravir should be approved.
Merck’s Molnupiravir has been monitored closely since data last month showed that when given at the early stage of infection, it could significantly decrease the chances of dying or being hospitalized for those most at risk of developing severe covid-19.

The drug, to be marketed as Lagevrio in the United Kingdom, is intended to introduce missteps into the genetic code of the virus that causes COVID-19 and is administered twice daily for five days.
So far countries across the world have heavily relied on covid-19 vaccines instead yet many vaccines proved to be inefficient including China’s CoronaVac which was sent back to China from various European countries after people who had taken the vaccine started to have severe effects and the vaccine did not stop the spreading of the virus.
This new covid-19 pill can be the game changer in eradicating the virus.
Also Read: PM Narendra Modi Meets Microsoft Co-Founder Bill Gates In Glasgow
2 Comments